Contact UsCONTACT
Please feel free to contact us if you have any questions or concerns.
Inquiry FormStories
STORIES
SERIES EMBARK

Sleep apnea syndrome (SAS) is a dangerous sleep disorder that causes patients to stop breathing during sleep and is a risk factor for cardiovascular and other diseases. Patients themselves have difficulty recognizing the symptoms and many feel uncomfortable wearing masks for continuous positive airway pressure (CPAP), the standard treatment for SAS, which has led to a low retention rate.
MaRI is developing a SAS treatment device that detects breathing and heartbeat during sleep using proprietary millimeter-wave radar technology and eliminates apnea with low-frequency sound stimulation. Since the treatment is non-contact and can be continued without feeling any burden, it is expected that more patients will be able to continue treatment.
We interviewed Hirofumi Taki, CEO of MaRI, about the therapeutic devices under development, how he came to start his own company despite being a doctor, and the important mindset of a manager.
(Interviewer: Chisae Fujiwara)
We are currently developing equipment to treat SAS.
The device has two parts: detecting apnea and treating it.
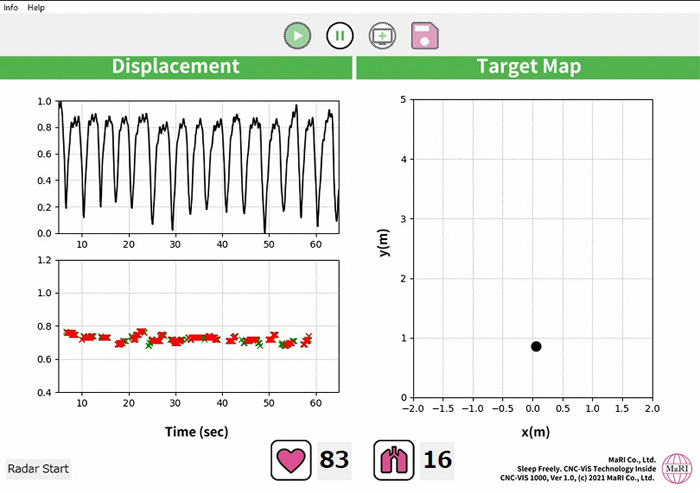
First, millimeter wave radar* is used for detection, and by taking reflections from the surface of the chest, the chest movement is used to determine if the patient has apnea. Once the apnea condition is detected, the next step is to stimulate arousal with low-frequency sound to a level that does not wake the patient, thereby encouraging normal breathing and improving the apnea condition.

Our strength lies in our technology that can detect apnea with a high degree of accuracy using millimeter wave radar. It is considered a very difficult technology to design a signal processing algorithm that can accurately detect respiratory status under any conditions, since the body is not in a constant orientation when sleeping and there are individual differences in the way the chest moves depending on the physique.
In general, people with SAS are known to snore a lot, but the most dangerous condition is actually when they stop breathing and wake up in a labored state. It is known that blood pressure spikes at this time, causing significant damage to the heart and blood vessels. Our device can accurately detect and treat apnea before it reaches such a state. In addition, this device uses low-frequency sound to provide non-contact treatment, so it does not place a burden on the patient. This has the great advantage of making it easier for patients to continue treatment.
We are currently conducting a clinical trial at Kyoto University Hospital to demonstrate the efficacy and safety of the treatment in SAS patients. Kyoto University Hospital has an organization called the Institute for Advanced Clinical Research and Development (iACT), which supports us in conducting efficient clinical trials.
In addition, we are also collaborative research with doctors in the cardiology and emergency departments. Since millimeter wave radar can monitor not only respiration but also heartbeat 24 hours a day, we hope to help detect early signs of illnesses such as myocardial infarction, stroke, and sudden infant death syndrome, and to elucidate their causes.
Our device also has the ability to monitor the patient's health at all times while taking privacy into consideration. Since receiving support from iCAP, we feel that our collaborative research with universities is progressing smoothly, and we hope that we can contribute to extending healthy life spans through further research in the future.
I was unsure about becoming a clinician when I entered Kyoto University School of Medicine and was in my sixth and final year. I had originally majored in physics rather than biology when I took the university entrance exam, and I wanted to develop medical devices. I wondered if I could create something that could solve a problem where a cure had not yet been established. So I consulted with a professor in my laboratory at Kyoto University, who introduced me to several companies, and I made a presentation on the medical device I wanted to develop, but was turned down. Looking back on it now, it was only natural (laughs). I felt at that time that "it would be difficult to develop products with people from a company from the research stage, so I should go to a graduate school of engineering to acquire engineering skills on my own. So I enrolled in the laboratory of Professor Toru Sato of the Graduate School of Informatics at the same university and studied digital signal processing and medical ultrasound technology.
After working as a specific assistant professor at the Graduate School of Informatics, Kyoto University, I had the opportunity to participate in a biodesign training program at Stanford University when I moved to Tohoku University as a specially appointed associate professor at the Graduate School of Biomedical Engineering. I saw a patient with SAS at the sleep clinic where I was assigned during that training, and I felt that it was very different from other diseases in that the patient had almost no subjective symptoms. The patient also visited the clinic after his spouse pointed out that he was snoring for the first time. We also found that although CPAP therapy, the standard treatment for SAS, is a very useful treatment device, many patients drop out of treatment halfway through because they feel it is too burdensome, and we felt there was a great need for it.
Stanford Biodesign does not allow you to choose which department you are assigned to, because the idea is that "there must be an unsolved problem in every department. It's peculiar, but I actually found these needs while I was there, so if I had gone to a different department, I would probably be making different medical devices now (laughs).

During the training, I learned not only how to conduct research and development with the goal of "delivering medical devices to patients," but also how to establish a company, how to gather research funds, how to obtain intellectual property, and everything else related to starting a business, so I was able to start without much anxiety.
In Japan, especially in university-based startups, the technology is often established first, then an application is found that fits the technology, and it is built from there.
But what I learned in the training was the importance of creating from "needs". Find a patient in need, find a place where you can get enough market, and then find a technology that fits the need. In other words, needs identification and product specifications are all done at the beginning of development. By doing so, we knew before development that we had a certain number of patients and marketability, and we knew the issues that needed to be solved to make the product worthwhile, so we were less anxious.
Yes, that has had a very big impact on us. It became very difficult to get access to hospitals, and it became very difficult to make progress in clinical research.
Conversely, however, we focused on using that time to further improve the accuracy of apnea detection. Originally, before the coronavirus infection epidemic, the detection accuracy of millimeter wave radar was not as high as it is now, and although it could generally detect the state of breathing, it was still insufficient in principle.
We also found a hospital where we could conduct a specific clinical study for healthy patients and completed Phase I (Phase I clinical trial). Because they were not patients, it was relatively easy for Corona Disaster to proceed with the clinical study. Therefore, we also adjusted the optimal volume of low-frequency sound for obese people who may be experiencing apnea, and checked the incidence of adverse events. We were able to confirm that the incidence of adverse events would not be a problem, since only 3 out of 60 subjects complained of a "slight headache" even when the sound volume was increased considerably.
Personally, I feel that entrepreneurship is similar to "chess". There are many different options and which one is closest to success - and which one is the most successful? We consider a great many possibilities. I try to plan far ahead, including backup plans, so that no matter which outcome is achieved, it will work.
In fact, when we first started the company, there was some talk about detecting the sounds that precede the apnea state, and there was even talk of taking apnea from the sound alone. However, there was a possibility that it might not work, so we placed millimeter wave radar as a backup plan. As a result, we realized that it would be difficult to detect complete apnea because there is almost no sound, so we switched to using millimeter-wave radar.
When I started my business, no one told me that I could start a business and make it work. It is natural because we are actually trying something new and everyone does not have the knowledge of what to do about it.
However, when you become a manager, you are the one who has the most information, so you have to formulate various strategies and make your own judgments about what you think would be a sure thing.
Of course, it does not mean that I do not listen to what others have to say, but rather, after listening, I digest it in my mind and input it as a piece of information while making my own final judgment. In return, I take full responsibility. In a sense, people who "go their own way" are suited for entrepreneurship, and I think their success rate will be high.
(Interviewed in October 2023. Affiliations, positions, etc. are as of the time of the interview)
MaRI is a medical device startup that develops devices to treat sleep apnea syndrome (SAS) using world-class digital signal processing technology as its core. MaRI's "gentle treatment" medical devices have been attracting attention. Kyoto-iCAP hopes that through MaRI's support, the issues facing patients and society will be overcome.

Masahiro Shinohara

MaRI Co., Ltd. Website
Please feel free to contact us if you have any questions or concerns.
Inquiry Form